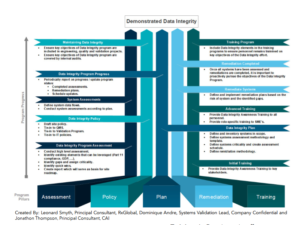
ABSTRACT
In 2018, the FDA released “Data Integrity and Compliance with Drug cGMP, Questions and Answers, Guidance for Industry.” Between 2015 and 2019, the number of 483 observations written globally increased by more than 10%. The increase indicates that either the industry is still catching up to compliance expectations or the FDA is inspecting with greater scrutiny. Not all of these observations are driven by the Data Integrity guidance, but the FDA has made it clear that this will be a focus area for inspections in the coming years. What are the basic principles of Data Integrity? How do you ensure that your facility is prepared for an inspection?