ABSTRACT
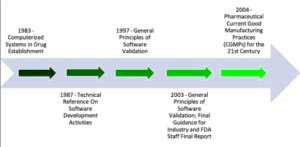
The Food and Drug Administration (FDA) has recently proposed dramatic changes to the current Computerized System Validation (CSV) approach that is being used by most companies in the Life Science Industry. Although not yet released, the proposed Computer Software Assurance for Manufacturing, Operations, and Quality System (CSA) guidance will assist manufacturers overcome several barriers to the efficient manufacture of drugs and medical devices. This will be accomplished through the use of implementing critical thinking early in the process, which will guide the verification process rather than using the industry’s current document-centric validation approach. The Computer Software Assurance guidance will provide manufacturers with the FDA’s point of view on the CSA process and will detail how industry can employ the process to verify a computerized system’s installation and operation. This white paper presents a short history of the FDA’s CSV guidance, how it became burdensome to projects, how the CSA approach compares to existing Agency guidance, and it presents steps that can be taken to implement the CSA approach to meet the goal of lowering the cost and duration of CSV efforts.