ABSTRACT
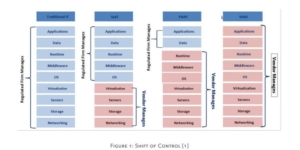
Software as a Service (SaaS) is a cloud computing delivery model that promises various benefits, but also comes with unique risks. The life science sector has very slow cloud adoption rate. One of the potential reasons for this phenomenon, as highlighted by GAMP Cloud Special Interest Group (SIG ) is the lack of regulatory and industry guidance specific to the subject. Having reviewed the available guidance, this paper proposes the main GxP considerations associated with implementation of a SaaS system, to support GxP process including manage, store, and archive GxP data, and resolves them into a conceptual risk-based model.